Cell and gene therapy is a tantalising space – precision medicine is undoubtedly the future of therapeutics. The cell and gene therapy industry is already making meaningful strides in treating rare diseases for small patient groups. The future requires scalability, and this will mean the potential to target not only rare but chronic, ubiquitous diseases for more patients.
These new targeted therapies can re-model a patient’s own cells to target rare diseases such as some blood cancers. Treatments such as CAR T-cells (chimeric antigen receptor T-cells) and technologies that can edit genes such as CRISPR/Cas9 have transformed modern day medicine. Many regulatory bodies such as the FDA are preparing for a wave of new cell and gene therapies and by 2025, between 10 to 20 new products are expected to be approved annually. There are more than 250 ongoing clinical trials studying CAR T-cells – not only to treat cancers such as melanoma, blood cancers, glioblastomas, etc., but to also investigate whether there is the potential to treat autoimmune diseases such as Alzheimer’s and dementia, which is the leading cause of death in the UK.
Whilst significant progress has been observed in the research and development space, there is much improvement to be made within supply chains to ensure that this growth potential is met. We are now at a crucial turning point, reminiscent of the ‘specialty drugs’ market two decades ago, where proper investment in cold-chain technology was necessary to fuel the market’s commercial success. The UK 2030 Life Sciences Vision states that in ten years’ time, CAR T-cell therapies, CRISPR and other DNA editing technology may all be deployed at scale if we focus on adequately scaling the supply chain.
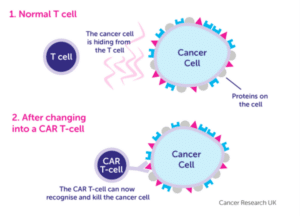
Source: Cancer Research UK
The complexities of scaling the supply chain
These therapies are currently aimed at small patient populations (low volume, high value and often made-to-order) and scaling has the potential to treat broader populations with chronic diseases more cost effectively. The supply chains needed to deliver cell and gene therapies are complex – there are multiple touchpoints and the personalised nature of these therapies leads to many variables and risks. To deliver these products at scale, the industry needs to adopt significant changes in manufacturing, automation, transportation, storage facilities and data tracking.
Cold-chain technology is an important requirement in the life sciences industry. However, scaling end-to-end cold chain processes (from manufacturing, storage and delivery) is complicated given the half-life of living cells is only a few hours. Specialist packaging is also required to be able to regulate temperatures as these therapies must often be transported in strictly regulated cold (or cryogenic) temperatures. Cell and gene therapy products are especially vulnerable to the ‘last mile’ of the supply chain when they need to be re-infused back into the patient.
Patient-centricity at the heart of advanced manufacturing
It is imperative that the patient is put first within the supply chain – ultimately, it starts and ends with the patient. Human cells are the first point of contact and the supply chain ends with the re-administration of these re-programmed cells back into the patient (cells are harvested and sent to laboratories over several weeks). As patients supply the raw material, their mixed backgrounds, disease stages and medical history introduces personalised complexity to production processes that is difficult to manage.
Between these start and end points, there are many moving parts with multiple sites and processes. Manufacturing complexity is what differentiates the cell and gene therapy field compared to traditional pharmaceutical manufacturing. Dealing with biological materials means that production batches are subject to higher risks of process deviations and failure to meet quality control leads to much higher patient consequences (synthetic chemicals are typically easier to predict and control).
Low volume, high variation products would typically use agile or adaptive lean methods of manufacturing. This can and does use automation but the key to automation in these environments is to enable ‘just-in-time’ manufacturing and flow. Manufacturing steps in cell therapy take variable amounts of time and bottlenecks can form with a fully automated approach. As an example, specific equipment may be used whilst the rest of the system sits idle, wasting time and resources. To add to the complexity, let’s remember: the vein-to-vein clock is ticking, CAR-T patients often undergo this treatment when all else has failed and the longer it takes for their treated cells to return, the higher the risk that the patient will not survive.
At present, most cell manufacturing processes utilise a ‘frozen-in-frozen-out’ process, whereby patient cells are frozen post-collection and manufacturing to prolong shelf-life and reduce supply chain variables (e.g. shipping delays). This means that time spent thawing samples (both at the start of manufacturing, and at the point of reinfusion back into the patient) extends the vein-to-vein time and potentially reduces the potency of the product.
Potential solutions to drive efficiency in the process may need to look at how to reduce the supply chain risk associated with a ‘fresh-in-fresh-out’ process (i.e. no freezing between sample collection and reinfusion). To achieve this, strong collaboration between manufacturing and distribution teams is required to ensure samples progress quickly between each step in the supply chain (as well as just-in-time and flow to remove any waiting at any processing step). Patient-centric manufacturing solutions must ultimately consider complex networks as well as complex processes.
Traceability is crucial: data integrity and traceability (chain of identity and custody) of the therapeutic product needs to be maintained throughout the supply chain. As each therapy is created for a specific patient, systems need to have the capability to track and verify the condition of the product and there are very few manufacturers today who can do this at scale. There are opportunities in this area to establish data standards as advanced manufacturing scales, whilst navigating a complex regulatory environment.
Supply chains will be the backbone to deliver growth
Cell and gene therapy is projected to grow at a CAGR of 18% from 2023 to2032. This is a changing field with ongoing clinical trials and huge growth potential, and supply chains will be crucial in scaling and delivering patient-centric therapies to treat diseases in the future.
Balancing patient-centricity with large scale manufacturing, whilst navigating a strict traceability environment may feel like a hard code to crack, but in reality, it needs creativity and discipline. Big pharma has often looked to its peers in Fast Moving Consumer Goods (FMCG) for examples on how to be more agile, and to Automotive manufacturers for process efficiency. Perhaps now is the time to consider looking at other sources of inspiration. An unlikely role model may be the luxury industry which deals with high-value low-volume items (many of which are distinct pieces) that have strict handling and storage requirements and must be carefully traced when moving around the world to prevent theft, loss, and damage. As the world of cell and gene therapy grows, those that can convert complex supply chain processes into reliable solutions will get ahead of the curve and thrive.
Author: Sophie Reid







